The Curia Hopkinton, MA facility is ISO 13485:2016 certified and provides GMP manufacturing of mRNA, antibodies, proteins, and vaccines and collaborates with Curia Hayward, CA for process development.
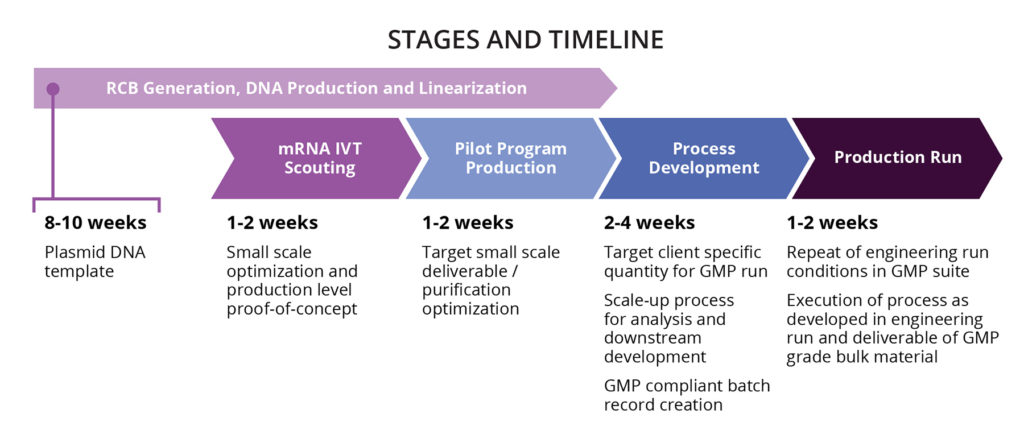
Curia Bulk Drug Substance mRNA Manufacturing Capabilities
- GMP compliant, single use equipment
- Cell-free mRNA manufacturing
- Large scale production up to 8-80 grams/batch
- ISO7 suite
- Expertise in self-amplifying mRNA production
- Onsite analytics for in-process testing and batch release
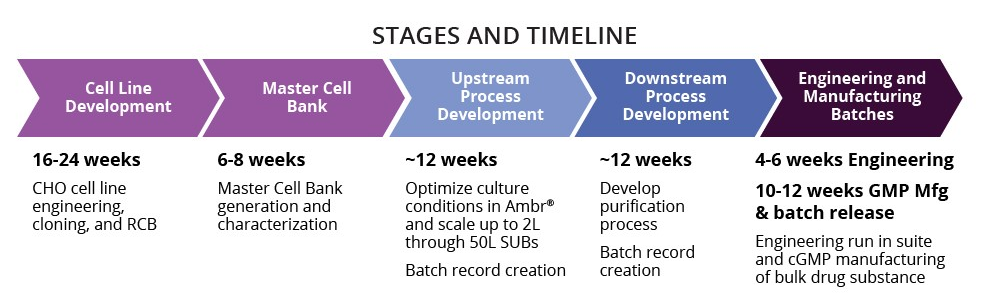
Antibody & Protein Therapeutic Development and cGMP Manufacturing
- Single use equipment
- 200, 500, and 2000 liter SUBs
- ISO7 post-viral and fill/finish suite
- Onsite analytics for in-process testing and batch release
- Total timeline ~18 months for phrase 1 drug substance
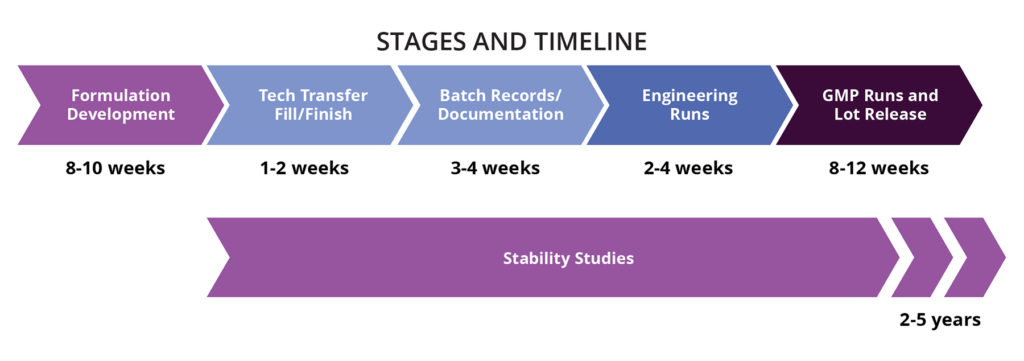
Drug Product Formulation Development and Manufacturing in Camarillo, Glasgow
- EU Grade A Environment
- Vial size: 2-30cc
- Fill Volume: 0.2-32 mL
- Max batch size:
- Liquid: 20,000 units
- Lyophilized: 1200-5,000 (2 cc to 20 cc)
- Onsite analytics for in-process testing and batch release
Download this presentation detailing our Biologics Development & Manufacturing Services.
Are you looking to accelerate your program or simply want to learn more about our services? If you would like to speak to a member of our expert team and arrange for a tour of our cGMP facility in Hopkinton, MA, please contact Curia here.